Imagine stepping into a world where precision and cleanliness aren’t just goals—they’re necessities. In industries like pharmaceuticals, biotechnology, and electronics, the integrity of your work depends on the environment in which it’s created.
This is where ISO and GMP compliance in clean room design take center stage. These standards are not just bureaucratic boxes to tick; they are vital benchmarks that ensure safety, quality, and efficiency. But why should you care? Because the success of your operations might hinge on these seemingly invisible guidelines.
By understanding their importance, you can enhance your processes, boost productivity, and protect your brand’s reputation. Dive into this article to discover how ISO and GMP compliance can transform your clean room from a mere space into a fortress of excellence.
Credit: www.kewaunee.in
Importance Of Compliance
Compliance with ISO and GMP standards is crucial in clean room design. These standards ensure safety, quality, and regulatory alignment. They are the backbone of a successful clean room operation. Ignoring compliance can lead to serious consequences. It can affect product quality and safety.
Ensuring Safety And Quality
ISO and GMP compliance ensures a safe environment. It minimizes contamination risks. This is vital in industries like pharmaceuticals and electronics. Clean rooms must maintain high standards. Contaminants can ruin products and pose health risks. Compliance acts as a safeguard. It ensures all processes are reliable and controlled.
Meeting Regulatory Standards
Regulatory bodies require strict adherence to standards. Compliance ensures your clean room meets these regulations. This is essential for product approval and market access. Non-compliance can lead to legal issues. It can also result in costly delays. Ensuring compliance protects your business and reputation.

Credit: www.cleanroom-industries.com
Iso Standards In Clean Rooms
ISO and GMP compliance ensures clean room safety and efficiency. These standards maintain controlled environments, vital for sensitive processes. Reliable designs reduce contamination, safeguarding product quality.
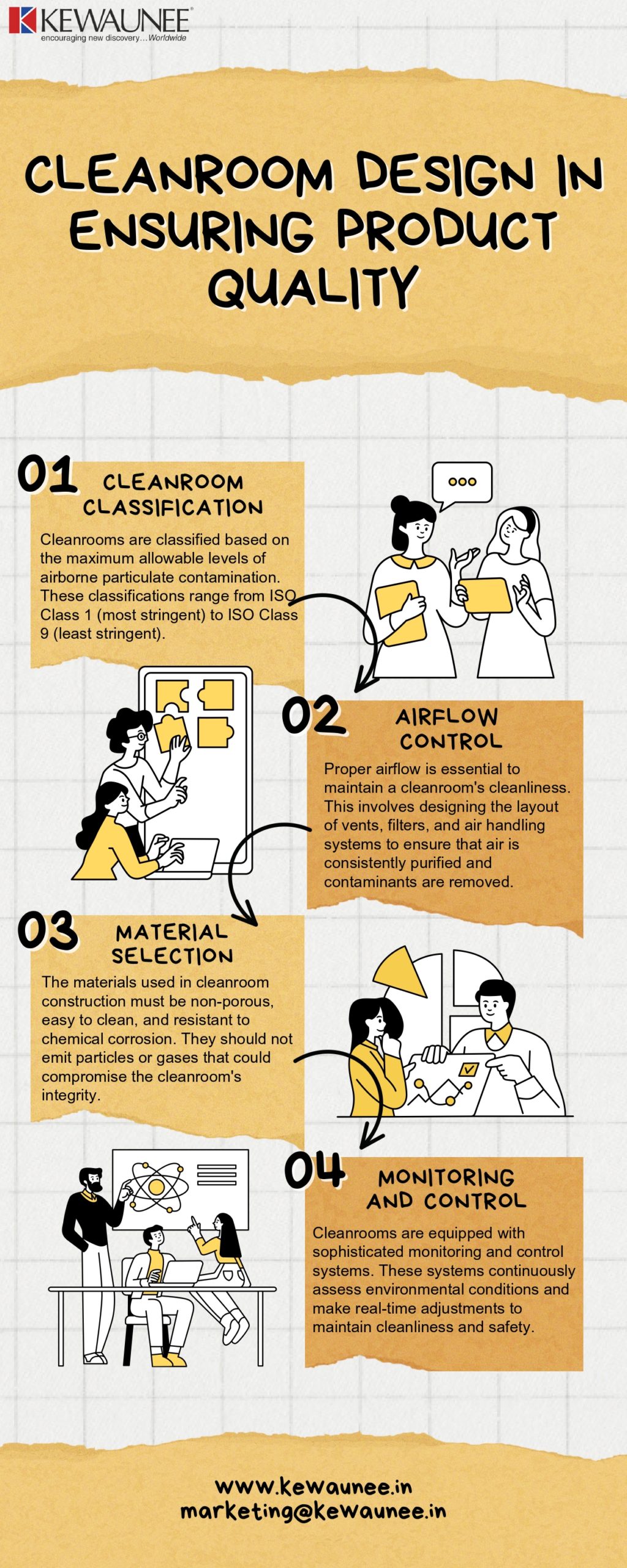
ISO Standards in Clean Rooms Clean rooms are essential environments where contamination control is critical. You might wonder why ISO standards are so crucial in these spaces. They ensure that the air quality and cleanliness meet specific criteria, safeguarding sensitive processes and products. Have you ever thought about how meticulous the air quality in a clean room must be to protect high-tech manufacturing or pharmaceutical production? That’s where ISO standards come into play.Iso 14644 Series
The ISO 14644 series is your roadmap to designing and maintaining clean rooms. It provides guidelines that help you achieve consistent cleanliness levels. Imagine walking into a clean room without these standards; the risk of contamination would be high, affecting your work’s quality. These standards also enable you to tailor your clean room design according to the precise needs of your industry, ensuring optimal performance.Classification Of Clean Rooms
Clean rooms aren’t one-size-fits-all. The classification system under ISO 14644 helps you determine the cleanliness level required for your specific application. Whether you’re working in pharmaceuticals, electronics, or aerospace, you need to know how clean your environment must be. This classification allows you to identify the number of particles permissible per cubic meter of air, providing a clear target for maintaining your clean room. Have you ever felt the pressure of getting every detail right in a high-stakes environment? This classification system helps alleviate that pressure by providing a clear framework. ISO standards in clean rooms are not just about compliance; they’re about achieving excellence. With these guidelines, you can safeguard the integrity of your processes and ensure that every product meets the highest quality standards. Have you considered the impact a tiny particle could have on your operations? ISO standards help you minimize such risks and elevate your work to new heights.Gmp Guidelines For Clean Rooms
Clean rooms are essential in various industries. They ensure product safety and quality. GMP, or Good Manufacturing Practice, guidelines are crucial in clean room design. These standards help maintain controlled environments. They reduce contamination risks and enhance product integrity. Understanding GMP guidelines is vital for anyone involved in clean room planning.
Principles Of Gmp
GMP principles focus on quality assurance. They ensure products meet safety standards. Clean rooms must adhere to these principles. They require strict control of air quality and temperature. Proper sanitation and hygiene are mandatory. Equipment must be regularly maintained and cleaned. Staff must follow specific protocols. Training ensures everyone understands their role in maintaining standards.
Impact On Pharmaceutical And Biotech Industries
GMP compliance is crucial in pharmaceuticals. It guarantees drug safety and efficacy. Clean rooms in this industry must prevent contamination. This protects patient health. Biotech firms rely on GMP guidelines too. They ensure biological materials are handled properly. These industries face strict regulations. GMP compliance helps them meet legal requirements. It builds consumer trust and confidence.
Design Considerations For Compliance
Designing a clean room that complies with ISO and GMP standards is vital. This ensures quality control and safety in various industries. Compliance demands attention to several design aspects. Key among these are material selection and airflow management. Proper design supports operational efficiency and reduces contamination risks.
Material Selection
Choosing the right materials is crucial for clean room compliance. Materials must be easy to clean and resistant to corrosion. Non-porous surfaces prevent microbial growth. Walls, floors, and ceilings should support sterilization. Durable materials extend the lifespan of the clean room. They also help maintain a controlled environment.
Airflow And Ventilation
Effective airflow is essential for maintaining cleanliness. Proper ventilation removes particles and controls temperature and humidity. HEPA filters are often used to ensure air purity. Airflow patterns should prevent contamination from entering clean zones. Regular checks and maintenance are necessary. This ensures the ventilation system operates efficiently.
Challenges In Achieving Compliance
Meeting ISO and GMP standards in clean room design is essential. These standards ensure safety and quality in manufacturing. Challenges include cost, training, and adapting to evolving regulations.
Designing a clean room that meets ISO and GMP compliance can be a daunting task. These standards ensure that clean rooms are built to maintain the highest quality and safety. However, achieving this compliance involves navigating several challenges that can affect your project’s timeline, budget, and overall success. Let’s dive into these challenges and understand how they impact your clean room design.Cost Implications
One of the primary hurdles in achieving ISO and GMP compliance is the cost. Meeting these standards often requires significant investment in high-quality materials, advanced technology, and expert consultations. This can quickly escalate project budgets, leaving you wondering if it’s all worth it. Imagine starting with a modest budget, only to find that the cost of compliant HVAC systems or specialized air filters is much higher than anticipated. This can lead to difficult budgetary decisions and potentially compromising on other aspects of your project. Are you prepared to make these tough choices?Technological Limitations
Another challenge is the technological limitations that you may encounter. While technology has advanced, not every innovation aligns seamlessly with ISO and GMP requirements. Sometimes, the latest tech gadgets promise efficiency but fall short of compliance standards, presenting a conundrum. Consider a situation where a new air purification system claims to be the best on the market. However, upon closer inspection, it doesn’t meet the specific particle filtration requirements set by ISO standards. This can lead to frustration and wasted resources. How do you ensure that your technology choices are both cutting-edge and compliant? Navigating these challenges requires careful planning, research, and a willingness to adapt. Are you ready to tackle these obstacles head-on to achieve a clean room design that not only meets but exceeds compliance standards?
Credit: www.mecart-cleanrooms.com
Benefits Of Compliance
ISO and GMP compliance play a vital role in clean room design. Adhering to these standards brings many benefits. They are crucial for maintaining quality and boosting business opportunities. Below, explore how compliance enhances product quality and increases market opportunities.
Enhanced Product Quality
Compliance ensures consistent product quality. It reduces contamination risks significantly. This leads to safer products for consumers. Quality control measures are more reliable. The production process becomes efficient and predictable. This ensures uniformity across batches.
Improved quality builds trust with customers. It encourages repeat purchases. Consumers prefer products they can rely on. Compliance means meeting high standards. This results in products that stand the test of time.
Increased Market Opportunities
ISO and GMP standards open up new markets. Many countries require these certifications. They act as a gateway to international trade. Without them, entry becomes challenging.
Compliance boosts reputation among industry peers. Companies with certifications are seen as reliable. This attracts potential partners and collaborators. Increased credibility leads to more business deals.
Having these certifications is a mark of excellence. It differentiates a company from competitors. Customers often choose certified products over non-certified ones.
Frequently Asked Questions
What Is The Difference Between Iso And Gmp Cleanroom?
ISO cleanrooms follow international standards for airborne particle levels. GMP cleanrooms focus on pharmaceutical production, emphasizing contamination control for product safety. Both ensure cleanliness but serve different industries.
Why Is Gmp Compliance Important?
GMP compliance ensures product safety, quality, and efficacy in manufacturing. It builds consumer trust and meets regulatory standards. Non-compliance risks legal issues and product recalls. It promotes consistent production and reduces contamination risks. GMP compliance supports business reputation and market access, ensuring safe products for consumers.
What Are The Gmp Requirements For A Clean Room?
GMP clean room requirements include controlled temperature, humidity, air quality, and contamination. Regular cleaning and monitoring are essential. Proper gowning protocols and equipment maintenance ensure compliance. Staff training is crucial for maintaining standards. Documentation of procedures and inspections is mandatory for transparency and accountability.
What Is The Iso Requirement For A Cleanroom?
ISO 14644-1 defines cleanroom requirements. It specifies cleanliness levels by particle count per cubic meter. Maintain temperature, humidity, and airflow. Regular monitoring and testing are essential. Compliance ensures contamination control in cleanroom environments.
Conclusion
Ensuring ISO and GMP compliance in clean room design is crucial. It guarantees safety and quality. Adhering to these standards builds trust with clients. It also improves operational efficiency. Proper compliance reduces contamination risks significantly. It enhances product consistency and reliability.
Designing clean rooms with these guidelines is smart. It meets industry requirements and legal standards. Businesses that prioritize compliance see benefits. They achieve better outcomes and stronger reputations. Investing in compliance pays off long-term. Success in clean room design starts with understanding these standards.
Prioritize them for a safe and effective environment.



